
Conductivity Test: Ohmmeter probes in a dab of ACZP show no conductivity...not in a 5" long snake or a 1/2" blob!
Anti-Corrosive-Zinc-Paste (ACZP) for Electrical Connections
ACZP or also Anti-Corrosive Zinc Grease ACZG are the author's generic terms for a family of products discussed in this tech article.
First published in Dec 2008 R. Kwas, Updated continuously [Comments with additional Info or emphasis added.]
--------------------
Is ACZP conductive?
Unique Metaphor
Reference Information
Suggested uses for ACZP
Fuse-ends
Twist-the-Fuse-to-fix-it Trick
Galvanic Chart
Comparison
of Zinc Dust filled Grease Products by Different Manufacturers
Links
ACZP success Story
Owners are invited to share their experiences/success stories!
--------------------
Recent posting of mine to the Brickboard.com 200 subforum:
"In an automotive environment with
temperature changes, condensation, out and out soaking by rain [and worse] water
under the hood, rubber boots, shrink tubing, tape, o-rings etc are all temporary
and not effective in the long-term, because they are not hermetic/gas-tight
seals...if you want a PERMANENT protection from environmental attack for your
electrical connections, use Anti-Corrosive Zinc Paste (NO! not dielectric
Grease!)... it'll protect your connections...including SUBMERGED (if that should
ever come up...ie. Chappaquiddick - keeping your electric windowlifts of you
Olds Delmont functional)! See:
http://www.sw-em.com/anti_corrosive_paste.htm
I've been using it and recommending it for all low voltage connections (not Ign
Hi-V) on the Brickboard 444-140 subforums for years - no decades!, and sending
it out with my upgrade kits for use on connections...no complaints yet!!...and
no intermittent electrical gremlins afterwards either! I've been convincing and
converting one vintage vehicle owner at a time...maybe you should consider using
it too...see link! "
--------------------
Background: To keep getting trouble-free service from electrical connections in our vintage vehicles, is pretty important. To help with this, I highly recommend the use of Anti-Corrosive-Zinc-Paste (ACZP, is my generic term). This is paste of conductive Zinc particles in a grease suspension, and should not be confused with insulating dielectric grease, which is often also recommended (maybe because the word dielectric has the word "electric" in it, so it must be good for 'em...WRONG!), but which is not the best solution. Why anyone would want to apply an insulating grease on a place where you want a good electrical connection is beyond me, because if this insulator actually got between the contacts, it would be degrading the connection, whereas when ACZP gets between contacts , Condition B (see below!) protection is taking place...read on...ACZP has two distinct advantages as it acts both microscopically as a sacrificial anode and superficially, creating a Gas-Tight-Joint (GTJ) to protect electrical connections....use it on new connections and when restoring old ones!
Zinc containing anti-corrosive pastes only protect an existing good electrical connection ...they will NOT improve a poor old connection (contrary to what one might wrongly take from their product info), so they sure as hell wont improve anything if slathered on the outside of a poor connection. Disassembly and a good cleaning are required first. This may be as simple as pulling off a 1/4" spade type terminal, and abrading any oxidation from the spade terminal (squeezing the mating terminal to restore a decent spring action...or better yet, and to be sure, replace the entire connector...apply paste to wire after stripping and before inserting into crimp - OPTIMAL! Link to: Making a Proper Crimp), also applying the paste onto the flat terminal, and reinstalling the connector. On more permanent connections such as the heavy current battery cable connections of the starter/solenoid, this requires a slightly more involved disassembly, but a cleanup to shiny metal is again required, then application of paste, and reassembly...the forces of the fasteners will displace the paste around the joint...excess can be wiped away.
Formulation viscosities range from Burndy's Penetrox A, a thick paste with very firm, almost putty consistency, which I have found will tenaciously stay where it is applied - even overhead or at elevated temperatures, to Ideal's much thinner Noalox which totally separates in the container, needing to be remixed before use...disappointing!...I much prefer the Penetrox...it's consistency does make it very difficult to coax out of the plastic squeeze bottle. I wound up cutting open the half full bottle and repackaging it to a much easier to use flat tin container. A toothpick or similar serves as a throw-away pinpoint applicator. More recently, I have discovered Ox-Gard by Gardener-Bender, and had a response by Ideal promising to improve the characteristics of their product.
LINK TO: Sw-Em ACZP Droop Test
LINK TO: Realworld Experiences with Electrical Corrosion and ACZP
Many different styles of connections are used on cars, some of these can be considered to be of a "high contact pressure design" (1/4" push-on, all bolted) but some of these connections must be considered a "low contact pressure design" (544, 122 and 1800E&ES Euro-Fuses, and especially the 3AG fuses used on the 1800; bayonet lamp sockets). In the "low contact pressure design" case, displacement of the grease from the current carrying area is not assured because of the low pressure, and certainly not as good as it might be, which can in fact make for a poor path for the electrical current, so this is NOT a good place to use the dielectric grease. Anti-corrosive paste, is, on the other hand perfectly suitable for ALL low voltage electrical connections (not High-Voltage Ignition)! There is no need to think about what kind of connection we are using it on! That is why I make the following statement.
Suggested uses for electrical anti-corrosive paste: Just about all automotive electrical connections that one wishes to get long-term, uninterrupted service from...including battery, chassis strap, fuse to holder, lamp to socket, antenna connections...etc. As a matter of fact, if anyone can come up with an automotive connection where it would not be suitable, I'd love to hear about it!
-------------------------
Is ACZP conductive?
Can (or should) it even be called "Conductive Grease"?
Answer: NO it is NOT, and should NOT be called that!
[Calling it "Conductive" on product data sheets is embellishment by the Marketing Dept. which the Engineering Dept, who knows calling it this is hokum(!), but was apparently not able to veto and correct! See also: Marketing Wankometer ]
As it consists of a grease base filled with conductive particles, ACZP is not conductive as none of the particles are in contact with each other. They are in fact surrounded by a non-conductive grease matrix. A simple test with an Ohm meter confirms this [See also "Unique Metaphor"!]:

Conductivity Test: Ohmmeter probes in a dab of ACZP show no conductivity...not
in a 5" long snake or a 1/2" blob!
Related Excerpt from a Forum Thread: http://www.volvoforums.org.uk/showthread.php?p=2040833#post2040833
"...is product (ACZP) compatible with all electrical metal interfaces? For instance I believe the battery post contacts would not be aluminum to same or to copper. Is there any interface where this product would be harmful." [A very valid question!]
My Response: "There is (thankfully) no
aluminum used in the electrical systems of our cars, only copper (wiring
and some terminals), brass (terminals), tin (terminals and plating
on some copper terminals), steel (studs for connections for ring
terminals and chassis), lead (Batt post and clamps)...I think that lists
most if not all of them...
ALL of these metals are compatible with and will benefit from the long-term
protection ACZP formulations give on reassembly (connection surfaces should be
clean and free of corrosion/contamination first!). Ox-Gard and Penetrox A are
suitable. Noalox is not." [See
below!]
[Note: As can be seen in picture above, AL/AL and AL/CU are listed on the Penetrox A product label, because these are the typical dissimilar connections an electrician will encounter...the additional dissimilar combinations we will encounter in vintage vehicles must be considered on the Galvanic Chart ...and I certainly have!...and we find that Zinc is lower on this chart that all of the metals which occur in the vehicles, meaning the zinc presence in the ACZP surrounding and between the connection will act to neutralize any corrosion which might start on those connections due to contamination present. ]
-----------------
ACZP is actually non-conductive! That's because the conductive zinc particles...more like zinc dust really, are suspended in non-conductive grease, and so are not in contact with each other...no direct conductive path actually exists across a blob of it (see above)...so it’s pretty benign stuff at the low voltages present a vehicle’s electric system, and should not be considered to be making electrical contact or carrying any current...and that’s why lathering it onto a connection without first separating and cleaning will only externally cover the connection and not take full advantage of the chemical benefit of the zinc particles...
When in place between and around two contacts which have mechanical pressure pushing them together, the benefits occur at the microscopic level. Three distinctly different conditions take place. To understand and appreciate the advantage, a finer inspection of these conditions is presented here:
ACZP at work!
Detail of ACZP at work!
Condition A - Direct Contact of Base Contacts. (Great...that’s the whole point!)...not much more needs to be explained here! These areas are in fact, the only current carrying areas! Without them, we’re in the dark!
Condition B - Base Contacts are Bridged by Conductive Particles: As the two contact surfaces touch each other either by sliding under substantial contact force (like the push-on connector shown), or by forcing the surfaces together (ring connector where a nut provides the high compression force, or even bare wire in a crimp), some of the particles are sheared or compressed, bridging the two surfaces. In this case, the zinc particles, being made of one of the least noble metals due to it’s low station on the nobility chart , (see: Galvanic Chart), and most willing to give up reactive ions, then act as zillions of sacrificial anodes which give up their ions, effectively passivating the area on a microscopic scale, while allowing the important base metal contacts to keep their ions and thereby remain undamaged by corrosion.
Condition C - Non-Bridging Particles in Suspension and Encapsulation: Since obviously most of the paste will be displaced, actually only a relatively small fraction of the zillions of tiny zinc particles will bridge the two base contacts. In fact, most will just be in suspension in the paste and will not be in physical contact with either electrical contact. These particles in the rest of the blob of grease which surrounds the connection don’t carry any current (see above), or give up any ions...as a matter of fact, they don’t do much at all besides take up space, and can be considered to just sort-of be along for the ride (kind-of like Steve G. in high school).
So the secondary benefit is realized from the Encapsulation as the soft paste is displaced to the next easiest place to be...right next to and surrounding the joint. This encases the joint in a protective coating which excludes moisture laden air in Oregon, or salty ocean air in Miami, or sulfuric acid droplets around the battery just about anywhere, from getting to the connection to do their dirty work and compromise it. The cycle of new moisture getting to the contact surface promoting new corrosion is broken. A Gas-Tight-Joint has been made. As noted before, with the creamy low viscosity of Ideal’s NOALOX product, the encapsulation benefit will be compromised as soon as the material runs away...and it will (from what my experience and semi-scientific but objective practical tests have shown. See: Sw-Em ACZP Droop Test )...how would you like it if your toothpaste ran off the brush? ...I personally would look for a new brand of toothpaste!
Link to Additional Information: Since I have used the 1/4" push-on terminal as an example here, the reader might be interested in this: The Lowly .250" Push-On Terminal
My Response to a Volvo Blog:
mkjtang; Please be aware that ACZP is not conductive as a blob, because the particles of zinc dust are all surrounded by a grease matrix...sort-of like the peanuts in the chocolate of a Mr. Goodbar, but on a smaller scale...the point is that until it is compressed between the contacts, it doesn't do much electrically or chemically [beyond protective encapsulation, Condition C above], but once compressed between the two metal surfaces, such that the zinc particles are in contact with the base contacts, they can do their beneficial chemical thing [Condition B above, plus "Unique Metaphor"]. Sort-of like your teeth mashing the chocolate aside until they contact and crush the peanuts [Condition C is chocolate, Condition B is peanuts...if you don't mash through it, all you taste is chocolate...only when you mash it together do the peanuts make themselves known!

Author's unique (and tasty) metaphor for why ACZP is non-conductive(!)
...and likely never to be seen in any engineering book!
...I have suddenly developed the inexplicable urge for a chocolate snack...!]
-----------------------------------------------------------
For those who think soldering terminals and wires is the best, IT IS! and you're right!...but applying ACZP is the next best thing!, because it also results in a Gas-Tight-Joint!
Crimps:

Applied to stripped wires before inserting into crimp.
[...out of a little container which I
repackage it into and send out with each SW-EM Kit. From the amount on the
stripped wires compared to what's in the little condiment container, it can be
seen that there is enough for many, many connections...maybe a whole car's
worth! See also:
ACZP Packaging ]
Push-on Connectors:

Applied to 1/4" push-on terminal tab and/or mating connector with a wooden
stirrer-stick.
Ring Terminals:

Applied to Ring term and/or its Stud...and yes it is also applied to every SW-EM
Alt Kit (2 pin) push-on connector!

Applied to Eurofuse ends and/or fuse terminals.
[Yes...twist the fuse after initial
installation...but ONLY ONCE, to seat it in place, displace the ACZP and
assure a firm contact, because you'll (likely) never have to concern yourself
with it again...yes, I said (likely) NEVER!]
Here's what might happen if you do the Twist-the-Fuse-to-fix-it Trick too often:
From Thread "Stubby fuse to bladed fuse": https://www.volvoforums.org.uk/showthread.php?t=329844&page=2 (Posting # 14):
"...removed the fuse on the problem circuit and discover(ed) a nice circular cut in both metal ends of the fuse where the ends fit into the little holes in retaining tabs...."
My response: "I suggest the circular holes were cut by the last owner doing the "simply twist the fuse in the holder" trick a few times too often with the ever present (when untreated) corrosion products acting as an effective abrasive and the holes (and non-contact) being the final result...a one-time application of ACZP would have prevented that too!"
-----
"Just Twist the Fuse"
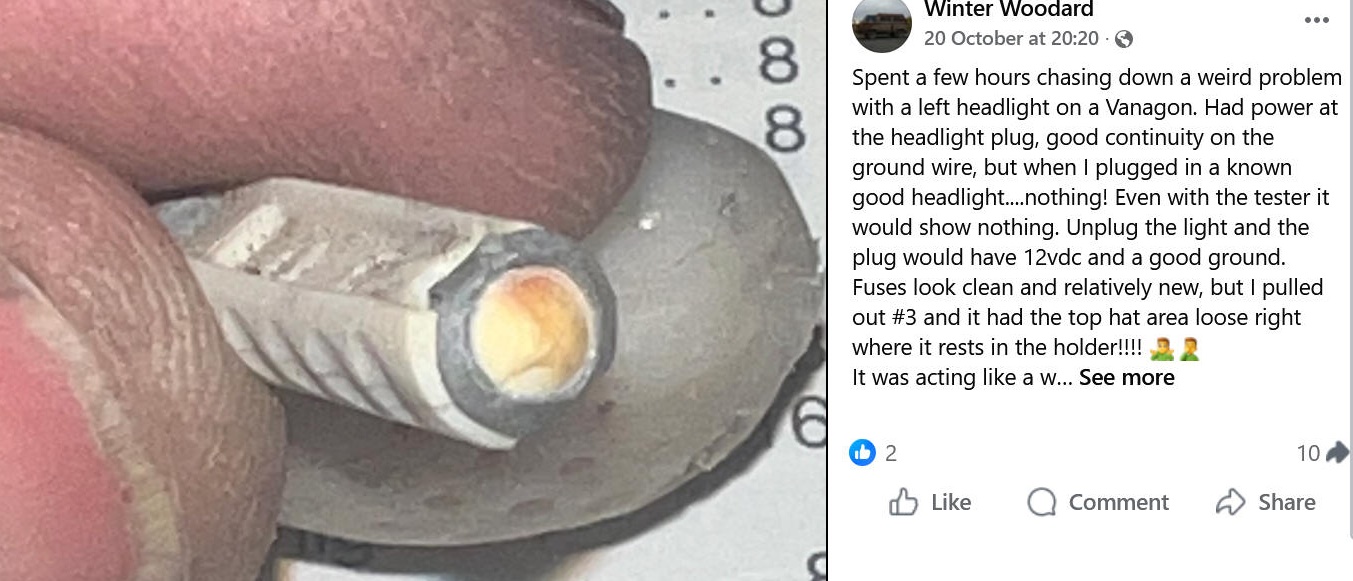
Copied from a ![]() posting on the Volkswagon Forum. Permission Requested
posting on the Volkswagon Forum. Permission Requested
My response to this posting:
" "loose top hat" is a good description of
what happens when the "just twist the fuse" trick has been used a few times too
often to restore operation, and the abrasive corrosion products have CUT that
"hat" away! [Resulting in a
hidden OPEN circuit!] I suggest applying Anti-Corrosive Zinc Grease and doing it just ONCE
and for THE LAST TIME after installing a new fuse!
The ACZG will prevent
corrosion and an intermittent connection forever more (yes, I said forever!!),
and you can move on to other things! Cheers from the vintage Volvo world, where
we hate electrical corrosion just as much as other vintage car enthusiasts! See
also: https://www.sw-em.com/anti_corrosive_paste.htm "
Bayonet Lamp-bases:

Applied to a previously cleaned brass lamp-base, including locking pins and main
contact. [Clean socket too! See
also:
https://www.sw-em.com/Amazon_Rear_Light_Fixture_Restoration.htm
(Bulb-Locking-Pin) and
Practical Application of ACZP]
Festoon Light Bulb Conical Endcontacts:
These can be found in the Courtesy and License Plate Lighting. Mechanically, they are held by spring force, between two End-contacts, not unlike a much smaller version at the Eurofuses.

ACZP on contact-area of a festoon lamp.
Placeholder for other suggested uses (no pix yet, but the reader should get the idea!) Battery Terminals, Chassis connections...etc.
"Before" Pictures:


A couple of examples of electrical connections from the VW world, exhibiting lots of
green or surface corrosion so (over-)due for an inspection of crimps, clean-up
and anointmentation with ACZP during
reassembly (at least)!
If you include a picture such as this [who knows how it looks, and what's happening, under there!!], with your posting to a forum asking about electrical problems, you are not listening to repeated advice, and so may possibly not have the correct attitude, or be worthy of owning a vintage car...you may need to consider selling it as soon as possible!
---------------

It can be seen that Zinc, because of its low station on this chart is one of the best metals for use as a sacrificial
anode...something which the maritime industry has known about, and taken
advantage of, for a long time! Contact with Zinc makes a "high"
galvanic voltage,
which is what drives the corrosion action, when in contact with almost every
other metal. Note from the chart, we're talking in the range of only
tenths of volts, but that is enough!
---------------
Comparison of Zinc Dust filled Grease Products by Different Manufacturers:
From Burndy's site (http://ecatalog.fciconnect.com/fci/datasheet.asp?PN=P8Ab&FAM=Penetrox&P=127610,127616):
Penetrox A Anti-Oxidant: Petroleum Base with suspended zinc particles

PENETROX oxide-inhibiting compounds produce low initial contact resistance, seal
out air and moisture, prevent oxidation or corrosion, exhibit superior
weathering characteristics, are usable over wide temperature ranges, and provide
a high conductivity "gas-tight"
joint. All PENETROX compounds contain homogeneously suspended particles. The
particles assist in penetrating thin
oxide films, act as electrical "bridges" between conductor strands, aid
in gripping conductor, improve electrical conductivity and enhance integrity of
the connection. The specially formulated PENETROX compounds are for use with
compression and bolted connectors providing an improved service life for
both
copper and aluminum connections. Additionally, the non toxic compounds are an
excellent lubricant for threaded applications reducing
galling and seizing.

Excerpt of Brundy's Tech Info sheet on the different Penetrox formulations.
[I recommend this product, as it meets all the requirements of providing long-term anti-corrosive protection for automotive connections, both protected, and exposed. Ron]
------------------------------------------------------
From Ideal's site (http://www.idealindustries.com/IDEAL-EZ/prodcat.nsf/Tables/Noalox?OpenDocument ):
Noalox® Anti-Oxidant Compound

Anti-oxidant and anti-seizing compound
Reduces galling and seizing on aluminum conduit joints
Suspended zinc particles penetrate and cut aluminum oxide
Carrier material excludes air to prevent further oxidation [It would be nice it it were of a consistency and viscosity which allowed the product to stay put, at the application site! That, as confirmed by the Droop Test linked above is why I recommend against the use of this product.]
Improves service life of aluminum electrical applications
For use with all types of pressure-type wire connectors
[I DO NOT RECOMMEND this product, as on initial review of description, it may seem to meet all the requirements, however, upon actual evaluation and practical testing, it has been shown its viscosity is much too low, such that it runs down from the point of application, making: 1. a mess of the area where it was not even applied, while: 2. NOT providing the advertised benefits. This has lead to additional testing and comparison (see also: Droop Test) after the completion of which, my dis-recommendation stands! Ron]
------------------------------------------------------
From Gardener-Bender's site: http://www.gardnerbender.com/en/ox-100b
Ox-Gard:

[I recommend this product, as it meets all the requirements of providing long-term anti-corrosive protection for automotive connections, both protected, and exposed. Ron]
------------------------------------------------------
My e-mail response and clarification to an email... "I thought the zinc in it made it a bit conductive." [A reasonable assumption, but not quite right! See above!]
The zinc particles are metallic and so conductive certainly, but since they are surrounded by a matrix of grease carrier [See also "Unique Metaphor"!], ACZP is not conductive per se, and it is not a general conductivity which brings with it the benefits...it is only when applied to contacts that benefits occur...considered more closely, there are three mechanisms happening:Response to a ![]() post on the VW Vanagon sub-forum about adding a (braided) Chassis Strap solving a
slow-cranking issues: "...added
a ground wire from a starter bolt to the underbody. Solved the issue. Had 2
other buses later, same thing, same fix."
post on the VW Vanagon sub-forum about adding a (braided) Chassis Strap solving a
slow-cranking issues: "...added
a ground wire from a starter bolt to the underbody. Solved the issue. Had 2
other buses later, same thing, same fix."
Braiding is good because as you probably are aware, the small individual conductor gauge is very tolerant of vibration and movement an engine to frame connection is subjected to...the issue is terminating it...big industrial crimped (or better yet, soldered) lugs are best, but simply doubled up and deflecting the strands around the securing/connecting bolt (with a fenderwasher with OD larger than braid width, so that it captures all the strands) will do it also quite adequately. Anoint the whole thing with Anti-Corrosive Zinc Grease, especially the terminations for long-term protection, and it will serve well for a long time, including exposed to the elements. (See: https://www.sw-em.com/anti_corrosive_paste.htm ) Cheers from the vintage Volvo world!
EDIT: What I forgot to add is that the tolerance of movement/vibration of braided wire is not absolutely necessary...by adding in a generous loop between the end termination or supports, a similar tolerance can (almost) be achieved using normal stranded wire...not optimum, but OK...as a super alternative, welding cable (heavy gauge, super small-gauge strands to accommodate high current and lots of movement in service!) can also be used very effectively. ...the stuff is expensive, but you don't need a long length! Protecting it with ACZP, still applies!
George Minassian picture of a Chassis to Engine Braid (to drool over!) used with his kind permission.

View from below, looking forward.
Chassis to Engine Braid clean and tight (and anointed with
ACZP), should be the target for these
important high current connections subject to vibration!
------------------------------------------------------
A Good Reference: Radio amateur K1TTT 's page for more good info on these products including pricing/sources: http://www.k1ttt.net/technote/antiox.html ]
Link to related thread (warm wires!): http://www.brickboard.com/RWD/volvo/1317588/140-160/fuel_pump_wire_connections.html
Link to another Radio Amateur's page on ACZP: https://www.w8ji.com/dielectric_grease_vs_conductive_grease.htm He is clearly an individual also with much technical and hands-on experience, but I don't so much like his presentation and explanation and comparison between dielectric grease, ACZP and its non-conductivity. I have tried to explain it here as best I could, and like my explanation better (reader shouldn't be surprised by this!), but his title already bothered me (..." dielectric-grease vs conductive grease", because as far as I know, there is no such thing as "conductive grease"...all grease is non-conductive and therefore dielectric!...we cannot and should not describe ACZP as this [see results of conductivity test above] , even though ACZP manufacturers sometimes do this!! in their product infos [...welcome to creative product description, and embellishment by the marketing department! See also: "Marketing Wankometer"] ...plus, he includes anti-seize and thermally conductive greases in his discussion products which have different specilized uses again, further muddying the issue...
Comments on his introduction: "
" Nonetheless, it's good to hear info and another explanation from a different source:
ACZP success Stories (Owners are invited to share their experiences/success stories!...I'd even like to hear experiences where the application of ACZG resulted in a less-than-successful situation!...I'm still waiting on that!) :
Feedback from a happy, and successful(!) troubleshooter:
Eugene T. writes:
"Wanted to share this little story with you.
Installed new turn switch as old one was physically broken inside.
Left works, right works. Do high beams work too? Oh no, they do not. Not only they don't but blue high beams light on the dash is veeeery dimly on. Weird! No relay clicking, no nothing
Ok let's see. Switch is brand new and seems like contacts touch when pulled and come apart when released so probably not that.
Ok let's check next thing down the line - headlights relay. Located it and removed from the mount to inspect connections. What is this? Yeacky, dirty, oxidized plugs. Could that be it?
Yes it could and it was!! After nice brushing contacts with small brass bristles brush and applying some of the "Ron's awesome electric paste" all of a sudden everything came alive! Relay clicked, High Beams came on and off as commanded by the switch, blue light showed full brightness when Highs were on and nothing at all when they were off - just like it was designed by Volvo back in the '73!
Figured you would like this little success story."
My response to his feedback
Eugene;
Thanks for relating this success story! Yes…it can be as simple as restoring the connections as originally intended…then protecting them forevermore with ACZP…!
-----------------------------

Besides the ACZP repackaged by SW-EM into little condiment tubs as seen above, handy little squeeze-packs of Penetrox A
are available by this manufacturer. This 1fluid oz.
is enough for many connections!
Culinary Safety Tip: Do NOT store this anywhere near packs of your favorite hamburger condiment (although the color coding, label, and flavor] should help prevent mix-ups!

Picture from the SW-EM condiments department.
------------------------------------------------------
This article is Copyright © 2008-2025. Ronald Kwas. The terms Volvo, Burndy, Penetrox, Ideal, Noalox, Gardener-Bender, Ox-Gard and Hershey's are used for reference only. I have no affiliation with any of these companies other than to try to keep their products working for me (or snacking on them!), help other enthusiasts do the same, and also present my highly opinionated results of the use of their products here. The information presented comes from my own experience and carefully considered opinion, and can be used (or not!), or ridiculed and laughed at, at the readers discretion. As with any recipe, your results may vary, and you are, and will always be, in charge of your own knuckles!
You are welcome to use the information here in good health, and for your own non-commercial purposes, but if you reprint or otherwise republish this article, you must give credit to the author or link back to the SwEm site as the source. If you don’t, you’re just a lazy, scum sucking plagiarist, and the Boston Globe wants you! As always, if you can supply corrections, or additional objective information or experience, I will always consider it, and consider working it into the next revision of this article...along with likely the unique metaphor and possibly wise-a** comment.